University of Toronto Solar Fuels Research Cluster
Nanostructured Solar Fuel Materials for a New CO2 Economy

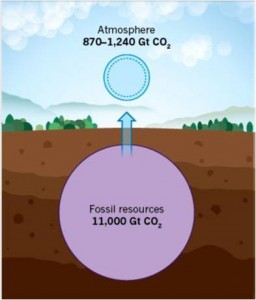
The cover art, courtesy of Chenxi Qian, illustrates the concept of how “black holes” for photons, phonons, excitons and molecules, in two new classes of nanostructure, allow light-assisted, gas-phase heterogeneous catalytic CO2 reduction to CO or CH4. The ultimate goal of this research is to facilitate an energy transition from today’s unsustainable “fossil fuel economy” of burn-and-adapt to greenhouse gas climate change to a new and sustainable “carbon dioxide economy”, where greenhouse gas emissions are utilized as a chemical feed stock rather than being treated as a waste product.
Two advances towards the vision of the “solar fuels refinery” of the future are portrayed in the front cover art that encapsulates the essence of two papers published in the new elite Wiley-VCH journal Advanced Science entitled “The Rational Design of a Single-Component Photocatalyst for Gas-Phase CO2 Reduction Using Both UV and Visible Light” published by Laura B. Hoch, Thomas E. Wood, Paul G. O’Brien, Kristine Liao, Laura M. Reyes, Charles A. Mims and Geoffrey A. Ozin, in Advanced Science 2014, 1: Article first published online: 29 DEC 2014 | DOI: 10.1002/advs.201470001 and “Photomethanation of Gaseous CO2 over Ru/Silicon Nanowire Catalysts with Visible and Near-Infrared Photons” by Paul G. O’Brien, Amit Sandhel, Thomas E. Wood, Abdinoor A. Jelle, Laura B. Hoch, Doug D. Perovic, Charles A. Mims and Geoffrey A. Ozin, in Advanced Science, 2014, 1: Article first published online: 25 NOV 2014 | DOI: 10.1002/advs.201400001.
UofT Solar Fuels Cluster: Assembled three years ago, this multidisciplinary team of faculty and students comprises the full spectrum of molecule and materials chemists (Geff Ozin and Doug Stephan); materials scientists and engineers (Doug Perovic, Zhenghong Lu, Ben Hatton, Chadra veer Singh); chemical engineers (Charles Mims and Cathy Chin); and an electrical and optical engineer (Nazir Kherani). Between them they have the combined experimental and theoretical expertise to (i) synthesize nanostructured materials, (ii) determine their structures and measure their properties, (iii) computationally model and guide experiments, and (iii) design and build photoreactors to evaluate their catalytic performance for the solar powered conversion of CO2-to-Fuel. If an economy could be built around CO2 as a chemical feedstock instead of treating it as a waste product, this would serve to stabilize the effects of climate change, provide long term energy security, and help to protect the environment. The UofT solar fuels cluster bring together a collective expertise that will allow them to succeed with this challenge and will lead to transformative research and extraordinary benefits to Canada
An Overview of Our Quest: Over the last few years our research has been successful in its aim to discover gas-phase, light-assisted heterogeneous photocatalytic materials and processes that can enable the solar powered conversion of CO2-to-Fuel [3,4,5]. With attainment of a high enough conversion rate and efficiency, these materials and processes will be developed into a solar fuels technology that will enable an energy transition from today’s unsustainable “fossil fuel economy,” with its associated risks of climate change caused by CO2 emissions, to a new and sustainable “CO2 economy”
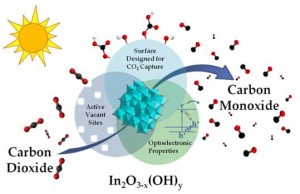
Figure 3 Illustration of key materials attributes of In2O3-x(OH)y nanocrystals that are considered responsible for their activity towards the photocatalytic reduction of CO2 to CO in a H2 environment.
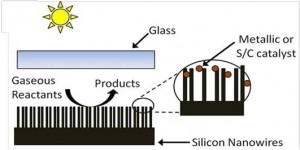
Novelty of Our Research: Recently the UofT solar fuels cluster has enjoyed some remarkable successes in converting CO2-to-Fuel using sunlight as the driving force with the discovery of two prototype systems that have significantly improved the outlook for research on solar fuels [3,4,5]. One advance involves the rational design of a single-component In2O3-xOHy photocatalyst for gas-phase CO2 reduction using both ultraviolet and visible light, Figure 3 [3,5]. The other entails photomethanation of gaseous CO2 over Ru/Si nanowire catalysts using visible and near-infrared photons, Figure 4 [4,5]. Both materials can be considered to behave as “black holes” for photons, phonons, excitons and adsorbed molecules enabling photocatalytic reduction of CO2 to CO or CH4 at high conversion rates.
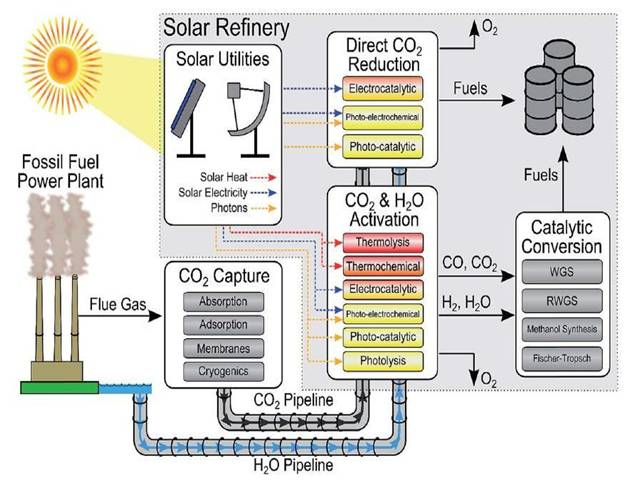
In a solar powered CO2-to-Fuel process, which can integrate readily into existing petrochemical infrastructure, it is the use of gaseous rather than aqueous reactants that distinguishes our investigations from mainstream solar fuels research around the world. In this context, the above mentioned breakthroughs brings the vision of a solar refinery a step closer to reality, Figure 2 [3,4,5]. To put these advances in perspective, earlier published work on solar powered CO2-to-Fuel over the past forty years has almost exclusively been performed by conventional aqueous-phase photoelectrochemistry and photocatalysis methods.
Conversion rates have been orders of magnitude below those of technological significance. Of the handful of papers that do exist on gas-phase solar powered CO2-to-Fuel, it seems they all suffered from artifacts arising from unsuspected adventitious carbon contamination of photocatalysts that at reported low conversion rates yielded products that were specious. Disentangling fact from fiction and resolving this serious problem demands the utilization of isotope labeled 13CO2 as reactant and detection, by gas chromatography-mass spectroscopy of organic products that must be authenticated by their diagnostic 13C isotope patterns. This is the only way to distinguish real-from-false positives. It was only in 2014 that the UofT solar fuels cluster solved this problem, simultaneously and independently with a research group in Spain and China [7,8], and published the first papers on gas-phase light-assisted CO2 photo-reduction using 13CO2, which yielded 13C isotope labeled organic products. This unequivocally proved the photo-reduction of CO2 was real and not an artifact. These breakthroughs represent the springboard from which our solar powered CO2-to-Fuel research evolved, providing the spark for the “revolutionary” phase to begin [3,4,5].
The objective of the proposed research of the UofT solar fuels cluster is to discover a new generation of high performance solar fuels materials and processes that will use CO2 together with sunlight, a source of H2 and a photocatalyst, for making renewable fuels such as CO, CH4 or CH3OH. This would constitute one of the modules in the envisioned solar refinery of the future, the structure and operation of which is illustrated in Figure 2 [6].
Solar Fuels Research Activities: The UofT solar fuels cluster will be involved in 8 major activities aimed at expanding upon and enriching the results of the “evolutionary” phase of their work outlined above to transition it to the “revolutionary” stage described above. These thrusts will comprise: (i) discovery, structure determination and property measurements of nanostructured materials active for light-assisted, gas-phase CO2 photoreduction; (ii) evaluation of conversion rates and efficiencies for production of solar fuels, such as CO, CH4, CH3OH by light-assisted, gas-phase heterogeneous catalytic reduction of CO2; and (iii) experimental and computational studies of surface chemistry, kinetics and mechanisms pertinent to these photoreactions. These studies will be complemented by (iv) optimization of materials catalytic performance; (v) developing material fabrication technologies for scaling; (vi) development and testing of prototype photoreactors; (vii) evaluation of the effect of solar concentration on CO2-to-fuel conversion rates, efficiencies, mass and energy balance; and (viii) life cycle process modeling to assess the material, energy and economic flows and hence the feasibility of making a solar fuels production facility from CO2 for the most active materials.
References: 1. www.ipcc.ch/; 2. Jakob, M., Hilaire, J., Nature, 2015, 517, 10; 3. L. B. Hoch, T. E. Wood, P. G. O’Brien, K. Liao, L. M. Reyes, C. A. Mims, G. A. Ozin, Advanced Science, 2014, DOI: 10.1002/advs.201400013; 4. P.G. O’Brien, A. Sandhel, T.E. Wood, A. Jelle, L.B. Hoch, C.A. Mims, G.A. Ozin, 2014, Advanced Science, 2014, DOI: 10.1002/advs.201400001; 5. G.A.Ozin, Advanced Materials, 2015, DOI: 10.1002/adma.201500116; 6. Herron, J.A., Kim, J., Upadhye, A.A., Huber, G.W., Maravelias, C.T., Energy and Environmental Science, 2014, DOI: 10.1039/c4ee01958j; 7. X. G. Meng, T. Wang, L. Q. Liu, S. X. Ouyang, P. Li, H. L. Hu, T. Kako, H. Iwai, A. Tanaka, J. Ye, Angew. Chem. Int. Ed., 2014, 43, 11478; 8. F. Sastre, A. V. Puga, L. C. Liu, A. Corma, H. Garcia, J. Amer. Chem. Soc., 2014, 136, 6798.
UofT Solar Fuels Cluster, Biographical Information
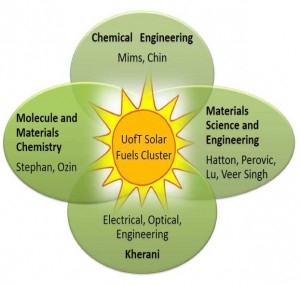
Venn diagram that illustrates the synergy and connectivity of expertise provided by members of the multidisciplinary UofT solar fuels cluster whose research is focused on solar powered conversion of CO2-to-Fuel.
The members of the UofT core solar fuels cluster comprise a multidisciplinary group of internationally renowned senior faculty and young up-and-coming faculty, who collectively provide a diverse set of synergistic skills that cross the boundaries of science and engineering at the UofT. The synergy and connectivity of research expertise is illustrated in the Venn diagram, the central target of which is solar powered conversion of CO2-to-Fuel to enable a new CO2 economy.
The UofT solar fuels cluster members include molecule and materials chemistry and nanochemistry (Stephan, Ozin, Chemistry), heterogeneous catalysis (Chin, ChemEng), reaction engineering (Mims, ChemEng), materials characterization (Perovic, MSE), computational strategies (Singh, MSE), device design (Hatton, MSE), photonics and optoelectronics characterization (Kherani, MSE-ECE), and science and engineering of metal-metal oxide heterostructures and energetics of molecules on these surfaces (Lu, MSE).
- Geoffrey A. Ozin – Distinguished University Professor, Canada Research Chair in Materials Chemistry and Nanochemistry, Department of Chemistry and Materials Science and Engineering, Lead PI. Ozin brings 45 years of research and teaching experience in materials chemistry and nanochemistry, skills that are invaluable for the discovery of solar fuels nanostructured materials. He will lead the effort on the synthesis, structure determination, property measurements and catalyst testing of nanostructured materials that can facilitate both gas-phase water photooxidation and carbon dioxide photoreduction.
- Doug Stephan, Professor, Canada Research Chair, Department of Chemistry. Stephan’s expertise is in synthetic chemistry and catalysis. In particular, Stephan has discovered and developed the chemistry of “frustrated Lewis pairs.” Such systems provide the ability of main group compounds to activate H2 and other small molecules and are directly relevant to mechanistic models for the surface mediated reduction of CO2. He will lead the effort on homogeneous catalyst systems that mimic those discovered by Ozin. As the reactivity and mechanistic aspects of homogenous analogs can be studied in detail, this will allow the synergy between homogeneous and heterogenous catalysis to be exploited for expeditious and systematic design improvements.
- Charles A. Mims – Professor, Chemical Engineering & Applied Chemistry. Mims has extensive experience in heterogeneous catalysis and reaction engineering from his previous work at Exxon Research and Engineering, and is the director of the newly established Ontario Centre for Characterization of Advanced Materials (OCCAM). He will lead the effort in solar fuels materials reaction engineering, designing highly precise methodology to quantitatively assess and optimize photocatalytic rates and efficiencies, scale-up strategies and techno-economic analyses of materials manufacturing and solar fuels photoreactor engineering.
- Cathy Chin – Assistant Professor, Chemical Engineering & Applied Chemistry – Heterogeneous Catalysis, Chemical Kinetics, and Reaction engineering. Chin will probe the mechanism of photocatalytic pathways of solar fuels materials based on isotopic transient, kinetic methodology, and in-situ spectroscopic techniques to establish the correlation between their atomic structures and photo-catalytic reactivity. She will also bring in strategies from her previous involvement at commercialization of micro-chemical reactors at Pacific Northwest National Laboratory.
- Doug D. Perovic – Professor and Celestica Chair in Materials for Microelectronics, Materials Science and Engineering. Perovic has extensive expertise in the chemical and structural characterization of nanoscale materials using state-of-the-art transmission electron microscopy and spectroscopy nano tools. He will supervise experimental efforts to fully characterize bulk and surface structure of the library of photoactive nanomaterials synthesized in Ozin’s group and fabricated in Lu’s and Kherani’s groups.
- Benjamin Hatton – Assistant Professor, Department of Materials Science & Engineering – Microstructural Surface Design and Control. Hatton has expertise in the design of surface microstructures, networks and devices. He will provide the development tools to engineer materials and devices to optimize surface area and porosity to control surface adsorption, mass flow and reaction efficiency of solar fuels materials.
- Chandra Veer Singh – Assistant Professor, Department of Materials Science & Engineering – Computational Materials Design. Singh will focus on understanding the theoretical underpinnings of the photocatalytic behavior of solar fuels nanomaterials that will enable computational discovery of novel material systems. He will utilize ‘first-principles’ calculations to investigate the band-gap energies and alignments of designed photocatalysts, the stable binding sites for molecular adsorption and determination of the reaction pathways and activation energies for the photooxidation and photoreduction mechanisms
- Nazir P. Kherani – Professor, Electrical & Computer Engineering and Materials Science and Engineering. Kherani’s expertise embraces photovoltaic materials, photonics and electronic devices.He will lead a team that brings considerable understanding and practical experience in the design and fabrication, electrical and optical measurement and testing of light harvesting optoelectronic materials and devices, invaluable for the development and evaluation of solar fuels materials and photoreactors.
- Zheng-Hong Lu – Professor, Department of Materials Science & Engineering. Lu’s expertise includes science and engineering of metal-metal oxide heterostructures and energetics of molecules on these surfaces. He will lead a team focusing on understanding the energies of molecules on catalyst surfaces and on the electronic structures at catalyst heterojunctions.